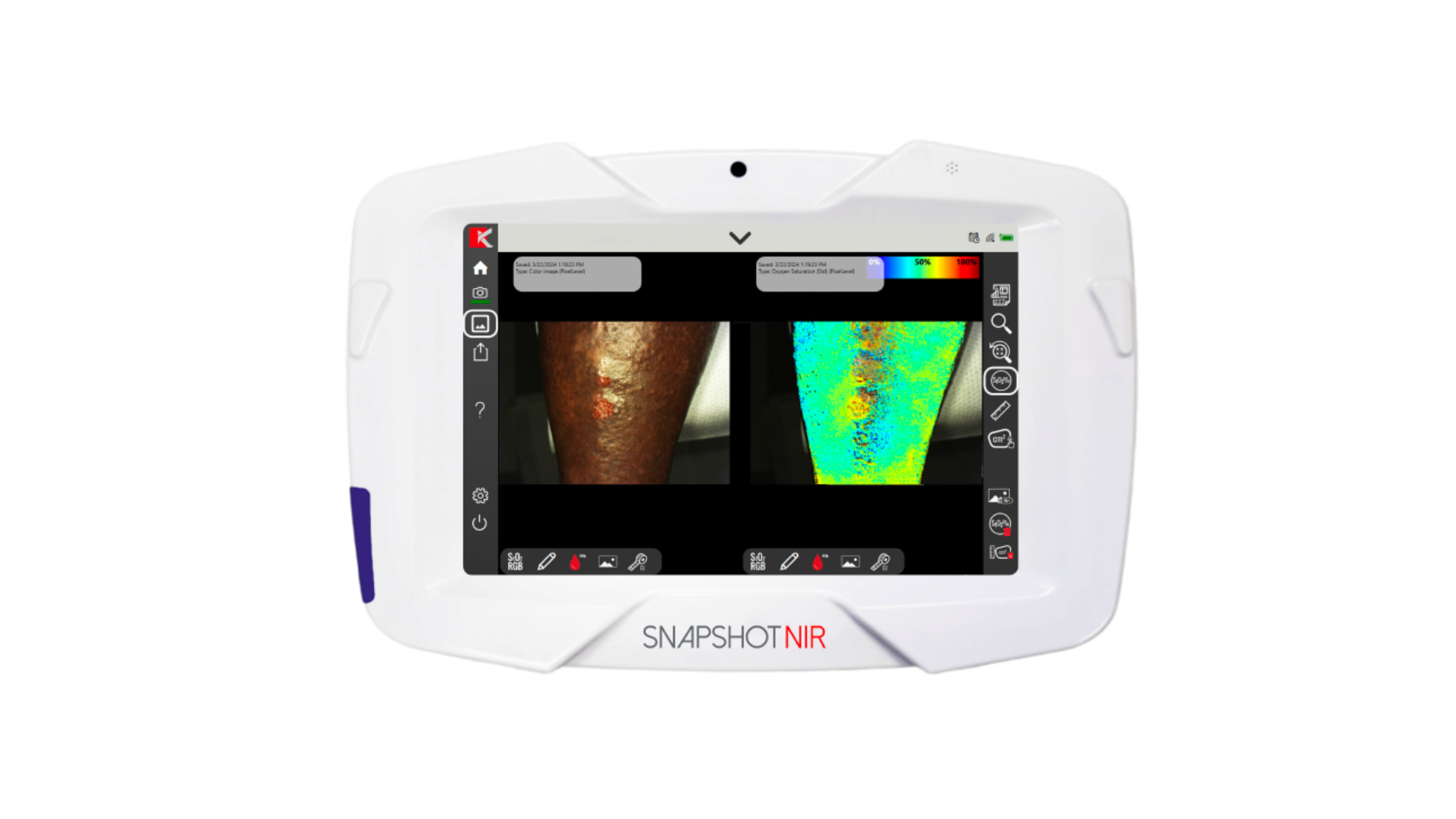
New Publication Reveals MolecuLight Imaging Significantly Improved Detection of Bacterial Burden Across Patients of All Skin Tones
April 26, 2023
Findings show that MolecuLight is an Objective Diagnostic Technology Positioned to Help Level Racial Disparity in Wound Care Outcomes
TORONTO, ON – (April 25, 2023) MolecuLight Inc., a manufacturer in point-of-care fluorescence imaging that locates and detects elevated bacterial loads in and around wounds, announced the publication of “Skin Pigmentation Impacts the Clinical Diagnosis of Wound Infection: Imaging of Bacterial Burden to Overcome Diagnostic Limitations”1 in the Journal of Racial and Ethnic Health Disparities. The publication addresses relevant and pressing issue of health care inequities as it applies to wound care, namely:
- A lack of clinical training and educational resources on diverse skin tones,
- An implicit/unconscious racial bias by providers, and
- A lack of diagnostic tools and approaches that address the unique clinical attributes of patients with different skin tones, most pronounced in those in the high pigmentation range.
The study involved a post hoc analysis of 350 chronic wounds from a prospective 14-site clinical FLAAG trial.2 It aimed to determine how the perception of clinical signs and symptoms of infection (CSS) differs depending on the patient’s skin tone and to determine whether fluorescence imaging technology, provided by MolecuLight, could offer a more objective diagnostic solution.
The study found that:
- Clinicians were far less likely to flag bacterial-laden wounds on highly pigmented skin as being problematic, highlighting the need for objective diagnostic solutions.
- The risk of racialized health inequities in wound care is higher when the diagnosis of pathogenic bacteria and infection are missed, resulting in treatment/intervention delays. This leads to an increased risk of complications and poorer outcomes. It has been well established in the literature that Black long-term care residents are far more likely to suffer from complications and die because of an infection than their Caucasian counterparts.3-5 Diagnostic limitations contribute to this inequity.
- Fluorescence imaging has great potential to serve as a more objective and equitable indicator of wound bacteria across all skin tones.
“The disparities in wound care in the US along racial lines is well documented and extremely disturbing”, says Dr. Alton Johnson, a co-author and Assistant Professor, University of Michigan Medical School, Ann Arbor, MI. “For example, studies report that pressure ulcers (or injuries) are more likely to form,3 are more severe,4 and are less likely to heal after 90 days in residents in Black nursing homes than patients in White nursing homes.5 This is unacceptable. This new publication explored the use of MolecuLight imaging and found that it is indeed an objective and equitable point-of-care technology that alerts the practitioner to high levels of bacteria on all skin tones. This enables intervention before infection sets in, increasing the chances of healing and helping avoid poor outcomes.”
“Acknowledging and raising both awareness and education around racial health disparities has been identified by clinical societies, associations, and committees such as the American Association of Wound Care as a critical first step in addressing this systemic problem”, says Dr. Jonathan Johnson, lead author and Founder/Surgical Director at Comprehensive WoundCare Services and Capital Aesthetic + Laser Center, Washington, DC. “In wound care, some of those disparities stem from our clinical assessment. We show in this study that clinical wound assessment is failing to flag the need for early bacterial infection management strategies in patients of color. Adoption of equalizing, objective technologies to address this root cause is in line with those awareness and education initiatives.”
“I treat wounds on patients of color every day and have seen firsthand the diagnostic challenges when erythema and other clinical signs are less apparent,” says Dr. Charles A. Andersen, co-author and Chief of Vascular/Endovascular Surgery at Madigan Army Medical Center Joint Base Lewis-McChord, WA. “MolecuLight technology can address this by objectively improving the detection of high bacterial loads otherwise missed during clinical assessment in all patients, as the intensity of red/cyan fluorescence signals is not impacted by skin tone. This represents a crucial step towards achieving health care equity for patients of color and may help reduce their disproportionally high risk of poor wound outcomes and amputation.”
The MolecuLight i:X® and DX are the only imaging devices for the real-time detection of elevated bacterial burden in wounds that are FDA cleared and CE and Health Canada approved. With clinical evidence including over 75 peer-reviewed publications involving 1,500 patients, they are used by leading wound care facilities globally.
References
- Johnson J, et al. Skin Pigmentation Impacts the Clinical Diagnosis of Wound Infection: Imaging of Bacterial Burden to Overcome Diagnostic Limitations. J Racial and Ethnic Health Disparities. 2023;
- Lam L, et al, Diagnostic Accuracy of Point-of-Care Fluorescence Imaging for the Detection of Bacterial Burden in Wounds: Results from the 350-Patient Fluorescence Imaging Assessment and Guidance Trial. Advances in Wound Care. 2021;10(3):123-136.
- Harms S, et al. Prevalence of pressure ulcers by race and ethnicity for older adults admitted to nursing homes. J Gerontol Nurs. 2014;40(3):20–6.
- Baumgarten M, et al. Black/white differences in pressure ulcer incidence in nursing home residents. J Am Geriatr Soc. 2004;52(8):1293–8.
- Bliss DZ, et al. Racial and ethnic disparities in the healing of pressure ulcers present at nursing home admission. Arch Gerontol Geriatr. 2017;72:187–94.
About MolecuLight Inc.
MolecuLight is the manufacturer of MolecuLight i:X®, a point-of-care fluorescence imaging device for digital wound measurement and detection of elevated bacterial burden in wounds, with CSS.
The views and opinions expressed in this blog are solely those of the author, and do not represent the views of WoundSource, HMP Global, its affiliates, or subsidiary companies.