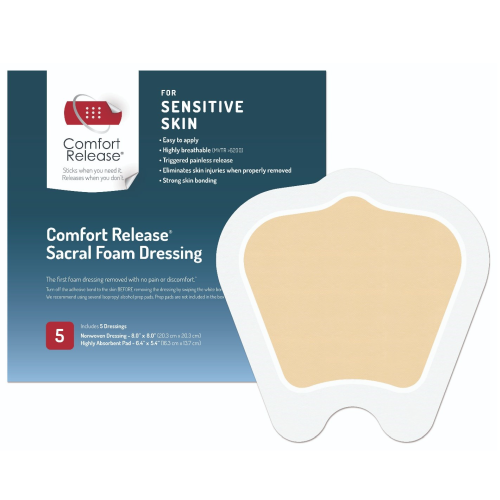
Comfort Release® Border Foam and Sacral Foam Dressings
Comfort Release® Border Foam and Sacral Foam Dressings' hydrophilic foam pad is made of soft foam that is comfortable, conformable and highly absorbent. Breathable and capable of expanding up to 50% when fully saturated. Made in USA.

Comfort Release®
Comfort Release® by Global Biomedical Technologies is a new, smart-switchable adhesive technology that addresses the pain and risk of injury in bandage and dressing removal. Comfort Release® products are available for advanced wound care, acute care, and everyday first aid.Benefits
• Absorbent hydrophilic foam for moderate to heavily exudating wounds
• Outer surface of the foam is bonded to a vapor-permeable non-woven breathable border
• Moisture resistant coating over the foam acts as a barrier to contamination to prevent infection and leaking of strike through drainage
• Lift and Look - can check on a wound without replacing the dressing
• Adhesive is "turned off" with a swipe of rubbing alcohol around the border for painless and trauma-free dressing removal
• Adhesion bond also tends to prevent maceration by inhibiting the lateral movement of exudate from the wound onto the surrounding skin
• Strong acrylic adhesive for long-lasting bond to skin
• Sacral foam designed to fit gluteal cleft
Indications
Comfort Release® dressings are suitable for dressing many types of wounds on patients with sensitive or fragile skin, including pressure injuries and traumatic wounds. The dressing absorbs exudate and maintains a moist wound healing environment while minimizing the risk of maceration when on and minimizing the risk of skin tears during removal.
Contraindications
The manufacturer has identified no absolute contraindications to the use of Comfort Release®, other than a known allergy to acrylic-based adhesives, or rubbing alcohol (used for dressing removal).
Warnings and Precautions
The presence of clinical infection does not preclude the use of Comfort Release® provided that appropriate antimicrobial therapy is also provided.
People with prior skin reactions to acrylic adhesives, skin burns or allergic reactions to rubbing alcohol should not use Comfort Release®. Do not reuse.
Storage Requirements
Store in a sealed pouch, in a cool and dry place at room temperature 59°F-86°F (15°C-30°C); avoid moisture and sunlight.
How Supplied/Sizing
Product features
Other features
Recommended Use
Acute Wounds
Burns
Chronic Wounds
Deep Wounds
Diabetic Foot
Granulating/Epithelializing Wounds
Infected Wounds
Moderate/Highly Exudating Wounds
Non/Minimally Exudating Wounds
Palliative Wounds
Pressure Ulcers
Sloughy Wounds
Superficial Wounds
Surgical Wounds
Venous Ulcers
Mode of Use/Application
Made especially for frail elderly, immunocompromised, or sensitive skin. Apply to clean, dry skin free of lotions, creams, or oils. You may use soap and water or alcohol prep pads to cleanse the periwound skin, then let it dry, prior to application.
Removal & Change Frequency
To remove, simply swipe the dry border area with rubbing alcohol – it is recommended to use one or two alcohol prep pads for each dressing. Large alcohol prep pads are enclosed in some product boxes or will need to be purchased separately. If needed, add more alcohol for painless release. After the alcohol evaporates, the adhesive bond returns and the dressing may be placed back onto the skin. This means you can lift part of the dressing to check a on a wound without having to remove the dressing. The interval between dressing changes should be determined by the degree of wound exudate produced; the dressing may be left undisturbed for several days on clean, lightly exuding wounds.
Construction
Comfort Release® Bordered Foam and Sacral Foam Dressings are made with a medical grade acrylic adhesive but can be used as a replacement for silicone adhesive dressings. Constructed with a plant-based polymer that allows for more secure adhesion to the skin but releases from the skin like a soft silicone adhesive (when removed as directed) painlessly and trauma-free.
Clinically Tested
Latex-friendly
Non-allergenic
Non-cytotoxic
Non-irritating
No posters match your selected filters. Remove some filtres, or reset them and start again.
Have a product to submit?
Be included in the most comprehensive wound care products directory
and online database.
Learn More











